Research | Remedy Institute
Remedy Institute is conducting a research study in collaboration with Toronto Metropolitan University and the Multidisciplinary Association for Studies in Psychedelics (MAPS) & MAPS Canada. Details on the study are below.
A Phase 2 Open-Label Treatment Development Study of MDMA-Assisted Cognitive Processing Therapy (CPT) for Posttraumatic Stress Disorder (PTSD)
Funding
This study is funded by a not-for-profit organization, the Multidisciplinary Association for Studies in Psychedelics (MAPS) & MAPS Canada
Purpose of the study
- This research study will combine Cognitive Processing Therapy (CPT), a well-known treatment for Posttraumatic Stress Disorder (PTSD) with MDMA.
- This is the first time these two therapeutic approaches will be blended together, making this an experimental study.
- This study is registered here.
FAQ
What is PTSD?
- PTSD is a mental health condition that can occur following a trauma
What are some possible signs of PTSD?
- Feeling the world is unsafe
- Upsetting memories
- Anger and irritability
- Loss of trust in others
- Feeling emotionally numb
- Avoiding upsetting memories of trauma
- Often feeling on “high alert”
- Difficulty sleeping or nightmares
Some sources of trauma can include*
- Physical or sexual abuse or assault
- Domestic violence
- Fire or natural disasters
- Racism and discrimination
- Racial profiling
- Assault by law enforcement
- Urban or gang violence
- Incarceration
- Pregnancy and childbirth experiences
- Ethnic cleansing and persecution
- Experiencing or witnessing torture
- Living in a warzone
- Immigration difficulties
- Deportation
- Military service
- Motor vehicle accidents
- Medical conditions and procedures
*Not everyone who experiences trauma develops PTSD. This does not mean the experience you had was not extremely challenging. PTSD is just one outcome that can happen after trauma.
What is CPT?
- CPT is one of the top treatments for PTSD.
- CPT helps individuals understand the impact that trauma has had on the way we think about ourselves, others, and the world.
- We are committed to providing CPT in a way that is respectful to people of all backgrounds and experiences.
What is MDMA?
- MDMA makes it easier to recall memories of the trauma and talk about the effects of trauma with less overwhelming feelings.
- MDMA combined with therapy has been shown to be effective for treating PTSD.
- MDMA is viewed as a therapeutic tool, rather than a “cure” for PTSD.
Is this treatment safe?
- To date over 1200 people have been given MDMA in controlled research settings.
- There have been no deaths, disabilities, or addictions as a result and no serious unexpected medical issues have happened.
- In this study, MDMA is administered twice within the 5 weeks of the study, unlike many medications for PTSD which need to be taken daily for years.
Approximate enrollment dates
- Enrollment is now closed.
Number of participants to be enrolled
- 10.
- Participants must live in Toronto, Canada.
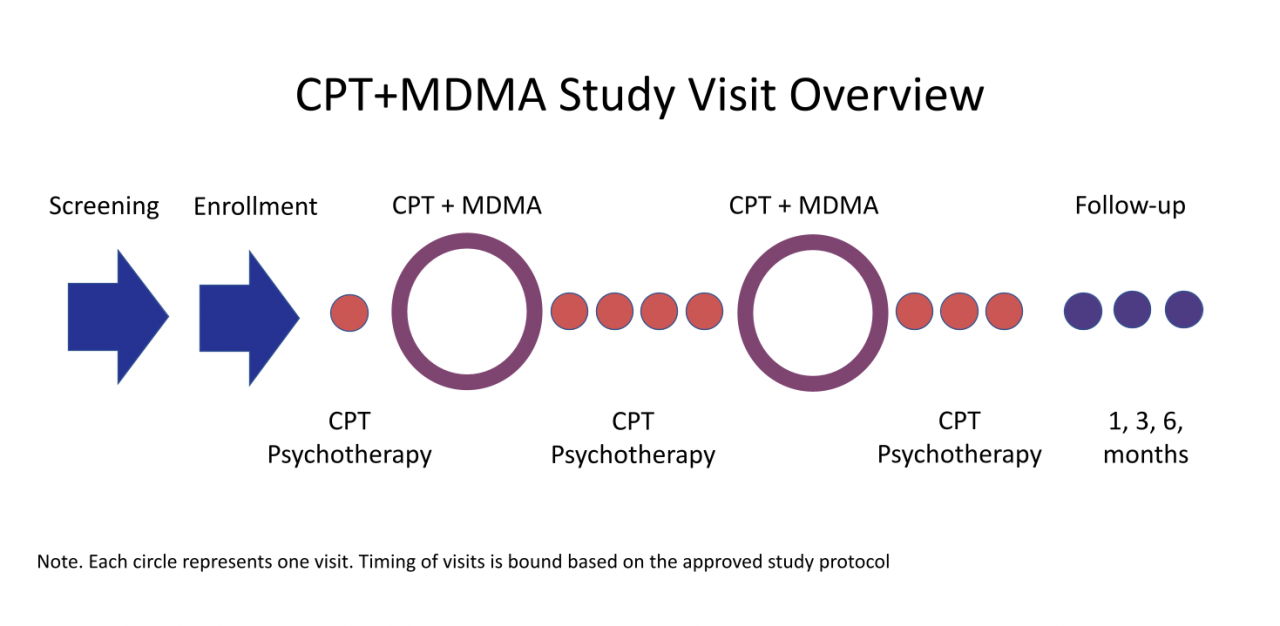
Study visit overview and commitment
- Interested volunteers can go through an initial eligibility screening questionnaire (link below).
- If you are deemed eligible you will be contacted to do a brief phone screen with study staff, consisting of questions related to mental health and medical history to assess suitability for the study. You will not be contacted if you are not eligible.
- If after the phone screen you are deemed eligible, you will meet with study staff for a thorough review of the study procedures and what your participation will consist of.
- There will also be assessments with a qualified researcher and a medical doctor that take place around this time to confirm eligibility.
- Participation in this study will include meeting with study therapists for 6 CPT psychotherapy sessions and 2 full-day CPT+MDMA sessions over 1.5 months.
- Preparation, CPT+MDMA and integration sessions will likely be conducted on Wednesdays, Thursdays and Fridays or, Fridays, Saturdays, and Sundays, respectively.
- Participants will complete questionnaires and meet with qualified researchers who will ask questions about their symptoms before they start the study, halfway through the study, and 1, 3, and 6 months after treatment ends.
We’re here
703 Bloor St. W, Toronto, ON

